The Dual Properties of Light
To ensure there are no misunderstandings; "light" as we will use it in this article, is defined simply as "illumination". Light radiates from its source outward until it reflects or bounces off of something. Think of light moving through space like water ripples moving through a pond. When light reflects off something red, the ripples that come back are more spread out. When it reflects off something blue, they’re less spread out and tighter together. Our eyes pick up on those differences instantly, and we interpret the distance as color.

Before 1905, the prevailing wisdom was that light didn't just act like a wave, it was a wave. The problem with wave theory is something called the photoelectric effect. In 1887, Heinrich Hertz built a simple transmitter and receiver to prove that electromagnetic waves (radio waves) existed. The transmitter was made of two metal balls with electricity applied to it. The metal balls had a space between them that allowed a spark to flow from one ball to the other. A few feet away, he had a loop of wire with its own small gap acting as a receiver. When a current was applied the transmitter sparked like it was supposed to. But sometimes the receiver would spark too. This showed the wave had traveled through the air and induced a current.

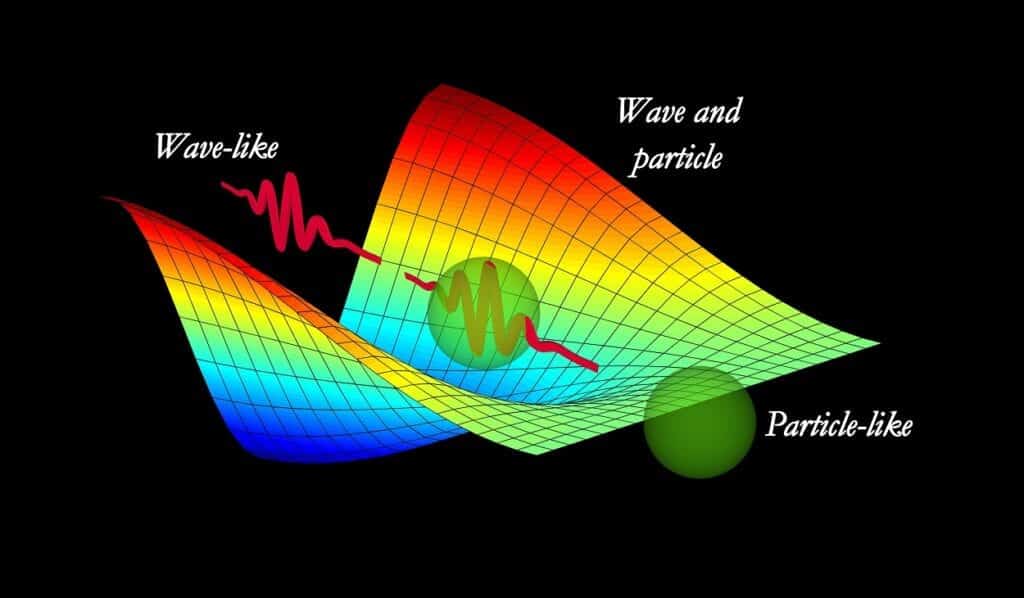
Hertz noticed that the receiver’s spark happened more easily when ultraviolet light was shining on it. In other words, UV light helped kick electrons loose from the metal, making it easier for a spark to jump across the gap. However, this only happened with UV light... not red or orange or any other color. This was a problem for wave theory. Wave theory said that the energy from light came from how bright the light was. The brighter the light, the more energy it would have. But no matter how high they turned up the brightness on other colors, they didn't loosen any of the electrons on the metal like the UV light did. Something else was going on here. It turns out, light is known is a "quantum" object. Meaning it has both the properties of a wave and the properties of a particle. UV light has shorter wavelengths than visible light, which means it has higher energy photons. The higher energy level of the UV light allowed the electrons to get knocked loose. So light is both a wave and a particle!
